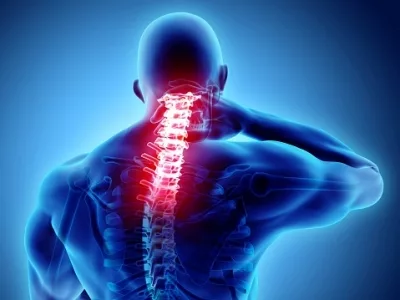
New York, April 1 (IANS) – Stem cell therapy has shown promising results for individuals with hard-to-treat traumatic spinal cord injuries, as per findings from a phase 1 clinical trial conducted by the Mayo Clinic in the US. The study revealed that stem cells derived from a patient’s own fat are safe and have the potential to improve sensation and movement in individuals with spinal cord injuries and paralysis.
According to neurosurgeon Mohamad Bydon from Mayo Clinic, “This study documents the safety and potential benefit of stem cells and regenerative medicine.” The trial, published in Nature Communications, included 10 adults, with seven showing improvements in sensation, muscle strength, and voluntary movement after receiving stem cell therapy. While the remaining three patients did not show improvement, their condition did not worsen post-treatment, with no serious adverse events reported.
The World Health Organization estimates that between 250,000 and 500,000 people worldwide suffer from spinal cord injuries annually, with limited treatment options available. Dr. Bydon emphasized that traditional treatments for spinal cord injuries have been limited to supportive care such as stabilization surgery and physical therapy. However, the potential of stem cell therapy in combination with other treatments could pave the way for a new treatment paradigm to enhance patient outcomes.
Despite the limited ability of the spinal cord to repair or regenerate cells, even slight improvements can significantly impact a patient’s quality of life. Dr. Bydon highlighted the importance of continued research into the use of stem cells for spinal cord injuries, stating that future studies may determine the effectiveness of combining stem cell therapy with other treatments to further improve outcomes for patients.
 Top 7 Causes of Tooth Decay
Top 7 Causes of Tooth Decay Buy Nicotine Pouches Online from Two Wombats: A Comprehensive Guide
Buy Nicotine Pouches Online from Two Wombats: A Comprehensive Guide The impact of health insurance
The impact of health insurance Community Health Initiatives: How Local Engagement Improves Overall Well-being
Community Health Initiatives: How Local Engagement Improves Overall Well-being Unlocking the Benefits of Medicare Part D Plans for 2025
Unlocking the Benefits of Medicare Part D Plans for 2025 Comparing Ascoril D Plus with other cough syrups and expectorants on the market
Comparing Ascoril D Plus with other cough syrups and expectorants on the market AstraZeneca’s Covishield To Cause Rare Yet Serious Side Effects
AstraZeneca’s Covishield To Cause Rare Yet Serious Side Effects Reinforce Your Smile: Discover the Benefits of Hydroxyapatite Toothpaste
Reinforce Your Smile: Discover the Benefits of Hydroxyapatite Toothpaste