[ad_1]
To verify for COVID-19 the Reverse Transcription Polymerase or RT-PCR and the Rapid Antibody Check out are utilized. Uncover out what they’re and the way in which it is used.
New Delhi: COVID-19 constructive circumstances have reached over 20,000 in India. The knowledge is simply not absolute as there is a lack of testing kits on the market out there available in the market and inside the nation, which is why testing is usually executed by the use of contact tracing. Contact tracing primarily based on WHO is intently watching the contacts of an contaminated specific individual to hint whether or not or not they have contracted the sickness so that they will get care and remedy if necessary, which is ready to cease extra transmission of the virus. The reason why India has opted for surveillance over mass testing is as a consequence of the shortage of testing kits on the market. There two vital strategies to verify whether or not or not a person has COVID-19- Reverse Transcription Polymerase or RT-PCR and the Rapid Antibody Check out to detect the presence of the virus.
How does testing work?
There two vital strategies to verify whether or not or not a person has COVID-19. The first being reverse transcription-polymerase chain response or RT-PCR, this verify takes nasopharyngeal swab or sputum sample. This verify can detect as little as one virus particle present in specific individual, nonetheless this verify can solely inform if the specific individual is at current contaminated or not. It cannot inform whether or not or not a person was contaminated earlier and has recovered. The verify outcomes can take wherever between a variety of hours to 2 days. The verify carried out by swab isn’t reliable because of the virus can disappear from the throat and multiply from the lungs. Due to this a sputum verify is much more environment friendly, significantly for a person testing on the second week from publicity.
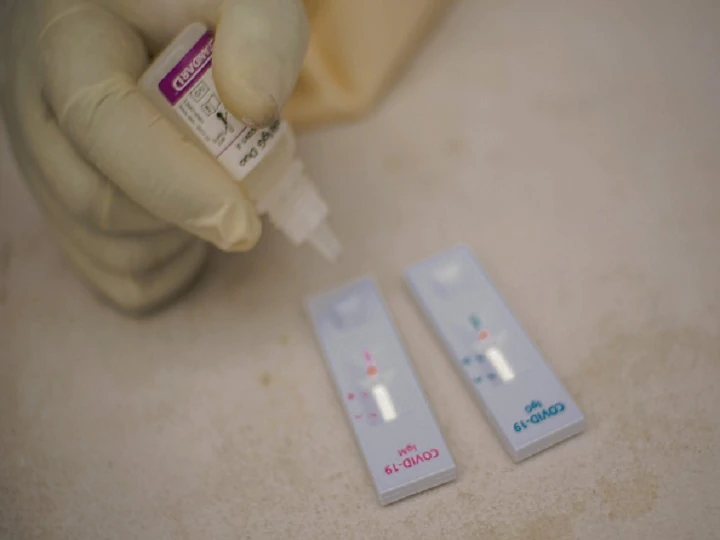
The second method is through Serology, which takes the blood sample. Immunoglobulin detection assessments are based on the qualitative detection of IgM and IgG which might be significantly generated by the physique in response to SARS-CoV-2 an an infection. IgG antibodies to SARS-CoV-2 sometimes change into detectable 10–14 days after an an infection although they may be detected earlier, and normally peak spherical 28 days after the onset of an an infection. Assays can be carried out in central laboratories (CLT) or by point-of-care testing (PoCT) aka Rapid Antibody Check out. The outcomes can take up to a few minutes to a couple of hours.
How does Rapid Antibody Check out work?
Rapid Antibody Check out works in an identical technique as a being pregnant verify nonetheless with a blood sample. Accumulate 2-Three drops of current blood/serum or plasma and place it in a sample container and place 2-Three drops of the buffer supplied in the an identical container (cassette). The cassette permits the diluted sample to maneuver by capillary movement. The cassette has SARS-CoV-2 antigen which is able to bind chemically with each IgM or IgG; thus, forming an antigen/antibody complexes of antigen/IgG and/or antigen/IgM and serving to to detect the presence of the virus as a result of it tells us if the physique is combating an an an infection. The verify tools has a administration line, a G line which reveals the presence of IgG (Immunoglobulin), an M line which reveals IgM (Immunoglobulin), and a unfavourable line which is ready to level out if the physique has no antibody. Often all through an an an infection, the physique’s first line of safety is with IgM immunoglobins whereas the IgG is produced later.
In step with an article inside the medical journal The Lancet ‘Antibody assessments are utterly completely different because of they require some data of the proteins that kind the viral coat—significantly, these proteins to which the immune system responds, triggering the manufacturing of antibodies that flag or neutralize the virus.’ Although the Rapid Antibody is simply not as appropriate as RT-PCR, they’re sooner. The PCR assessments work on DNA nonetheless as a result of the coronavirus is an RNA virus it is first reworked to DNA which takes time. That is the explanation Rapid Antibody testing is extraordinarily environment friendly in contact tracing or testing in large volumes as an illustration in hotspots. The ICMR considers this verify as a supplementary verify which will level out the presence of a sickness in a selected house. In step with some research, India continues to be import testing kits and since there is a worldwide demand for these kits India like many nations is working in want of these kits. The ICMR has solely authorised only one homegrown tools, although assessments are being carried out to make testing rather more setting pleasant and quicker.
[ad_2]
 PM Vishwakarma Yojana 2024: How to Apply Online; STEP-BY-STEP Guide
PM Vishwakarma Yojana 2024: How to Apply Online; STEP-BY-STEP Guide Pradhan Mantri Surya Ghar Yojana 2024: Scheme Details, Eligibility, Subsidy and How To Apply Online?
Pradhan Mantri Surya Ghar Yojana 2024: Scheme Details, Eligibility, Subsidy and How To Apply Online? BJP Youth Wing President Tejasvi Surya Nominated His Second-In-Command, Abhinav Prakash For A Debate With Rahul Gandhi
BJP Youth Wing President Tejasvi Surya Nominated His Second-In-Command, Abhinav Prakash For A Debate With Rahul Gandhi Haryana Free Scooty Yojana 2024: How to Apply Online?
Haryana Free Scooty Yojana 2024: How to Apply Online? Char Dham Yatra 2024 Starts: Online Registration, Routes, And Everything Important
Char Dham Yatra 2024 Starts: Online Registration, Routes, And Everything Important At UNGA, India Votes In Favor Of Palestine’s Bid To Become Full Member
At UNGA, India Votes In Favor Of Palestine’s Bid To Become Full Member Poonam Kasturi, Social Entrepreneur And Founder Of Daily Dump, Popularly Known As Compostwali, Dies At 61
Poonam Kasturi, Social Entrepreneur And Founder Of Daily Dump, Popularly Known As Compostwali, Dies At 61 Bhupendra Jogi Gets Attacked by Knives, Suffers Serious Injuries
Bhupendra Jogi Gets Attacked by Knives, Suffers Serious Injuries